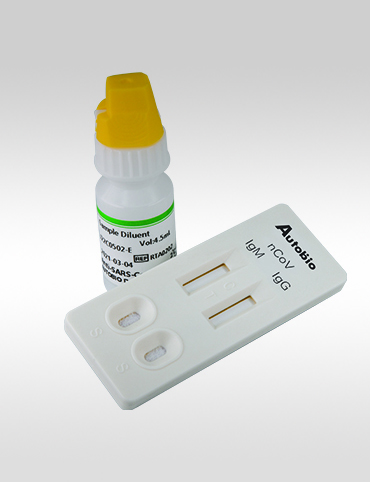
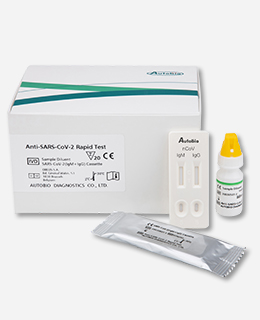
AutoBio Anti-SARS-CoV-2 Rapid Test
A RAPID ONE-STEP ANTIBODY TESTAnti-SARS-CoV-2 Rapid Test is a rapid, one-step lateral flow assay intended for the qualitative detection and differentiation of IgM and IgG antibodies to the SARS-CoV-2 virus in patients suspected of a COVID-19 infection. By using a patient’s serum, or plasma specimen, the Anti-SARS-CoV-2 Rapid Test offers a turnaround time of only 15 minutes.
DESCRIPTION
The Anti-SARS-CoV-2 Rapid Test is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human plasma from anticoagulated blood (Heparin/ EDTA/ sodium citrate) or serum from individuals with signs and symptoms of infection who are suspected of COVID-19 infection. The Anti-SARS-CoV-2 Rapid Test is also intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection.
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C 263a, to perform moderate or high complexity tests. The Anti-SARS-CoV-2 Rapid Test is intended for use by clinical laboratory personnel specifically instructed and trained in the techniques of in vitro diagnostic procedures.
Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in recent contact with the virus, due to the lag time between exposure and the patient’s antibody response.
This test should not be used as the sole basis for patient management decisions. Test results must be combined with clinical observations, patient history, and epidemiological information.
Follow-up testing with a molecular diagnostic test should be considered to confirm the infection status. This test is not intended for the screening of donated blood.
-
-
This kit is FDA Emergency Use Authorization (EUA) Approved
Features and Benefits
- Results in 15 minutes
- No special equipment needed
- Easy-to-interpret results
- Built-in-controls
- Room temperature storage
The FDA is not aware of an antibody test that has been validated for diagnosis of COVID-19 infection. While FDA remains open to submissions of these tests for such uses, based on the underlying scientific principles of antibody tests, we do not expect that an antibody test can be shown to definitively diagnose or exclude COVID-19 infection. As stated in the Policy for Diagnostic Tests for Coronavirus Disease-2019, validated antibody tests offered under the policy in that guidance should, among other things, include in test reports information that negative results do not rule out COVID-19 infection and that follow-up testing with a molecular diagnostic should be considered to rule out infection, and should be ordered only by clinicians who are familiar with the use and limitations of the test.
SPECIFICATION
- Positive agreement rate: 97.4%
- Negative agreement rate: 96.2%
- CE-IVD. For in vitro diagnostic use.
- Product no: RTA0202 / RTA0203
- Package size: 20 cassettes / 50 cassettes
- Sample type: human serum, plasma or whole blood
- Time to result: < 15min
Related Products
-
LOOKING FOR RESULTS?
Tiger Testing does not provide test results over the phone, email or tawk chat to individuals. If you were tested in Georgia or North Carolina and got a text from Tiger Testing (Radeas Labs) , please call 919-263-1150 or visit https://www.radeas.com/contact-us for test results. This company is not associated with us.




BENEFITS
Studies suggest that antibody testing for COVID-19 may provide useful information in managing the infected patient, making determinations as to immune status, and preventing the future spreading of the disease.