Quidel Solana SARS-CoV-2 ASSAY System
ACTIONABLE MOLECULAR RESULTS IN 25 MINUTES.FDA Emergency Use Authorization
The Solana SARS-CoV-2 Assay is an isothermal Reverse Transcriptase - Helicase-Dependent Amplification (RT-HDA) assay intended for the qualitative detection of nucleic acid from SARS-CoV-2 in nasopharyngeal (NP) and nasal (NS) swab specimens from individuals suspected of COVID-19 by their healthcare provider.
Molecular identification of SARS-CoV-2 occurs by the use of targeting highly conserved regions of the SARS-CoV-2 virus non-structural Polyprotein (pp1ab).
The assay consists of two major steps: (1) specimen preparation, and (2) amplification and detection of target sequences specific to SARS-CoV-2 using isothermal Reverse Transcriptase — Helicase-Dependent Amplification (RT-HDA) in the presence of target-specific fluorescence probes which is performed in the Solana instrument.
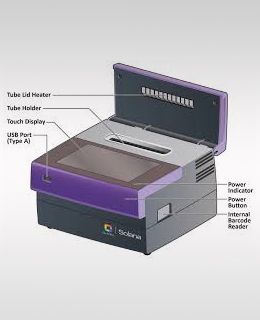
Results are displayed on the touchscreen, can be saved to the instrument, printed, and are capable of being sent to the LIS and exported through one of Solana’s five USB ports. Solana SARS-CoV-2 Assay is also supported by the power of Virena®.
DESCRIPTION
Solana is a molecular testing solution for COVID-19 without the tradeoffs between throughput, lengthy turnaround times, highly complex workflows, and reagent availability. Quidel’s Solana instrument and robust respiratory testing menu now features the Solana SARS-CoV-2 Assay. With Solana, we offer a scalable and flexible molecular solution that delivers highly accurate molecular results in an actionable timeframe.
Solana is a bench top instrument that combines Quidel’s proprietary helicase-dependent amplification (HDA) with fluorescence detection to deliver molecular results you can trust, faster than ever before.
The Solana testing system is easily accessible and can be seamlessly integrated. Solana’s workflow is easy and flexible, capable of testing a single specimen or batching up to 11 tests at a time.
-

-
Key features & Benefits:
Feature Benefit RT-HDA technology
Rapid method of isothermal nucleic acid amplification that does not require thermal cycler
Frozen Master Mix
Easy-to-use format, just thaw Master Mix before use (see Package Insert)
Small, easy-to-use instrument
Eliminates the need for dedicated molecular space and costly capital equipment, allowing for testing in smaller labs
CLIA moderately complex
Requires minimal training
Storage
Process Buffer 2˚C to 8˚C storage, Master Mix -70˚C or below
Long shelf life
6 months from date of manufacture
Room temperature set up
No ice or cooling block required
The Solana SARS-CoV-2 Assay is an isothermal Reverse Transcriptase - Helicase-Dependent Amplification (RT-HDA) assay
intended for the qualitative detection of nucleic acid from SARS-CoV-2 in nasopharyngeal (NP) and nasal (NS) swab
specimens from individuals suspected of COVID-19 by their healthcare provider. Testing is limited to laboratories certified
under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, to perform high or moderate
complexity test.
SOLANA SARS-CoV-2 ASSAY SPECIFICATION
- Sample Type Nasal swabs and nasopharyngeal swabs in transport media* obtained from patients suspected of COVID-19
- Time to Results Approximately 30 minutes
- Reagent Storage Conditions Process Buffer Reaction Tubes 2˚C to 8˚C and Master Mix -70˚C or below
- Controls Storage Conditions 2°C to 8°C
- Sample Preparation STorage Conditions Specimens should be collected, transported, stored, and processed according to CLSI M41-A. Specimens should be stored at 2°C to 8°C until tested. Specimens collected in BD/Copan UTM are stable at room temperature (RT), 2°C to 8°C or -70°C or below for up to 4 days. Specimens collected in the CDC Viral Transport Medium should be stored at 2°C to 8°C for up to 72 hours after collection.
- PPA Nasal: 97.2% Nasopharyngeal: 100%
- NPA Nasal: 100% Nasopharyngeal: 96.9%
Related Products
-
LOOKING FOR RESULTS?
Tiger Testing does not provide test results over the phone, email or tawk chat to individuals. If you were tested in Georgia or North Carolina and got a text from Tiger Testing (Radeas Labs) , please call 919-263-1150 or visit https://www.radeas.com/contact-us for test results. This company is not associated with us.









BENEFITS
Positive results may be detected in as little as 5 minutes
Negative results in 13 minutes
Molecular technology targeting COVID-19 RdRp gene
Designed for near patient testing in a variety of healthcare environments1
Room temperature storage
Direct sample types include: Nasal, Throat, and Nasopharyngeal swabs
Facilitates effective patient management